Microwave assisted synthesis and QSAR study of novel NSAID acetaminophen conjugates with amino acid linkers†
Abstract
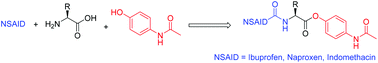
Novel, non-steroidal anti-inflammatory drug (NSAID), acetaminophen conjugates 6a–l with amino acid linkers were synthesized utilizing benzotriazole chemistry. Biological data acquired for all the novel bis-conjugates showed (a) some bis-conjugates (6d, 6e, 6h, and 6k) exhibit more potent anti-inflammatory activity than their parent drugs, (b) the potent bis-conjugates show no visible stomach lesions in contrast to parent drugs which are highly ulcerogenic, and (c) that the potent bio-active compounds have no mortality rates or toxic symptoms at 5 fold the applied anti-inflammatory dosage. A statistically significant QSAR model describing the anti-inflammatory properties of 6a–l (N = 15, n = 3, R2 = 0.891, R2cvOO = 0.770, R2cvMO = 0.796, F = 29.904, s2 = 0.011) was obtained employing CODESSA-Pro that validated the observed bio-activity.

 Please wait while we load your content...
Please wait while we load your content...