The origin of exo-stereoselectivity of norbornene in hetero Diels–Alder reactions†
Abstract
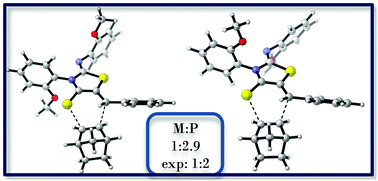
In this study the exo selectivity in the hetero Diels–Alder reaction of atropisomeric 5-benzylidine-2-arylimino-3-aryl-thiazolidine-4-thiones with norbornene was investigated with computational tools. Taking into account the M/P chiral character of the o-methoxyphenyl substituted heterodienes in addition to the exo/endo selectivity, 8 different transition structures were located. Based on the direction of approach of the diene and the dienophile for each plausible path it is found that endo products are not preferred because of the large distortion of norbornene and the rather eclipsed conformations of these transition state structures. Computational results are consistent with the experimental exo/endo selectivity. The computational methodology (M06-2X/6-31+G(d)//B3LYP/6-31+G(d)) was justified by comparison of the experimental rotational barriers with the calculated ones for selected compounds.

 Please wait while we load your content...
Please wait while we load your content...