AcOH-mediated dichloroimination of indoles using chloramine-B: a facile access to 2,3-functionalized indolines†
Abstract
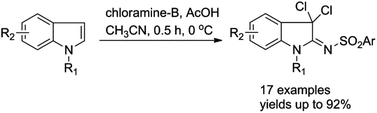
A new and mild method for the efficient synthesis of 3,3-dichloro-2-sulfonyliminoindolines via AcOH-mediated dichloroimination of indoles using chloramine-B is presented. Application of this method to the efficient construction of pyrrolidinoindoles and N–C3 linked pyrrolidinoindolines is demonstrated.

 Please wait while we load your content...
Please wait while we load your content...