A new approach to asymmetric synthesis of infectocaryone†
Abstract
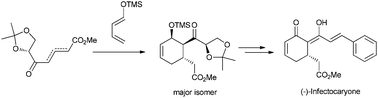
A useful and flexible strategy for synthesis of (−)- and (+)-infectocaryone from commercial sugars is developed. The key step of the synthesis is a new-type Diels–Alder reaction with good chemoselectivity and stereoselectivity, in which a mixture of alkene regioisomers in a dynamic equilibrium is employed as chiral dienophiles for the first time.

 Please wait while we load your content...
Please wait while we load your content...