Pyrazole–oxadiazole conjugates: synthesis, antiproliferative activity and inhibition of tubulin polymerization†
Abstract
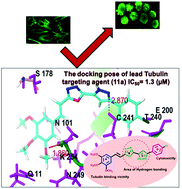
A number of pyrazole–oxadiazole conjugates were synthesized and evaluated for their ability to function as antiproliferative agents on various human cancer cell lines. These conjugates are comprised of pyrazole and oxadiazole scaffolds closely attached to each other without any spacer as two structural classes. The Type I class has a trimethoxy substituent and the type II class has a 3,4-(methylenedioxy) substituent on their A rings. Among these conjugates, 11a, 11d and 11f manifest potent cytotoxicity with IC50 values ranging from 1.5 μM to 11.2 μM and inhibit tubulin polymerization with IC50 values of 1.3 μM, 3.9 μM and 2.4 μM respectively. The cell cycle assay showed that treatment with these conjugates results in accumulation of cells in the G2/M phase and disrupts the microtubule network. Elucidation of zebrafish embryos revealed that the conjugates cause developmental defects. Molecular docking simulations determined the binding modes of these potent conjugates at the colchicine site of tubulin.

 Please wait while we load your content...
Please wait while we load your content...