Azide–alkyne cycloaddition for universal post-synthetic modifications of nucleic acids and effective synthesis of bioactive nucleic acid conjugates†
Abstract
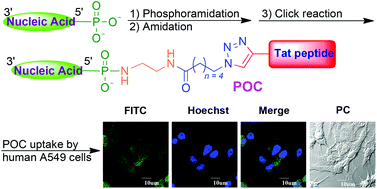
The regioselective post-synthetic modifications of nucleic acids are essential to studies of these molecules for science and applications. Here we report a facile universal approach by harnessing versatile phosphoramidation reactions to regioselectively incorporate alkynyl/azido groups into post-synthetic nucleic acids primed with phosphate at the 5′ termini. With and without the presence of copper, the modified nucleic acids were subjected to azide–alkyne cycloaddition to afford various nucleic acid conjugates including a peptide–oligonucleotide conjugate (POC) with high yield. The POC was inoculated with human A549 cells and demonstrated excellent cell-penetrating ability despite cell deformation caused by a small amount of residual copper chelated to the POC. The combination of phosphoramidation and azide–alkyne cycloaddition reactions thus provides a universal regioselective strategy to post-synthetically modify nucleic acids. This study also explicated the toxicity of residual copper in synthesized bioconjugates destined for biological systems.

 Please wait while we load your content...
Please wait while we load your content...