Efficient asymmetric synthesis of N-protected-β-aryloxyamino acids via regioselective ring opening of serine sulfamidate carboxylic acid†
Abstract
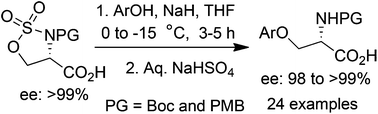
First regioselective ring opening of serine derived cyclic sulfamidate by hard nucleophiles like ArONa is developed, where β-elimination of serine sulfamidate ester by stronger nucleophiles is overcome by reversal of the electronic effect of the carboxylate anion. This method provides easy and direct access to a variety of N-Boc- and N-PMB protected β-aryloxy-α-amino acids with complete retention of enantiopurity in moderate to high yields.

 Please wait while we load your content...
Please wait while we load your content...