Metal-free oxidative olefination of primary amines with benzylic C–H bonds through direct deamination and C–H bond activation†
Abstract
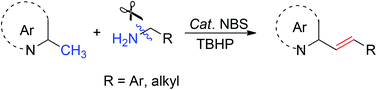
An oxidative olefination reaction between aliphatic primary amines and benzylic sp3 C–H bonds has been achieved using N-bromosuccinimide as catalyst and tert-butyl hydroperoxide as oxidant. The olefination proceeds under mild metal-free conditions through direct deamination and benzylic C–H bond activation, and provides easy access to biologically active 2-styrylquinolines with (E)-configuration.

 Please wait while we load your content...
Please wait while we load your content...