A theoretical study on the mechanisms of the reactions between 1,3-dialkynes and ammonia derivatives for the formation of five-membered N-heterocycles†
Abstract
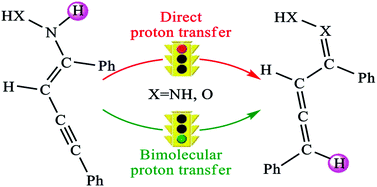
The reactions between 1,3-dialkynes and ammonia derivatives (such as hydrazine and hydroxylamine) for the formation of five-membered N-heterocycles (i.e. 3,5-disubstituted pyrazole and 3,5-disubstituted isoxazole) have been investigated using the density functional theory (DFT) method. The calculated results indicate that the favorable mechanism of this kind of reaction generally contains four processes: (1) the Cope-type hydroamination reaction between the reactants coupled with the hydrazine/hydroxylamine-assisted proton transfer process or the trimolecular hydroamination reaction via a six-membered transition state, (2) the bimolecular proton transfer process for the formation of an allenyl oxime intermediate, (3) the cyclization process, and (4) another bimolecular or hydrazine/hydroxylamine-assisted proton transfer process to afford the final products (3,5-disubstituted pyrazole and 3,5-disubstituted isoxazole). The computational results demonstrate that the novel bimolecular proton transfer process occurs in a stepwise manner and the first step of the novel bimolecular proton transfer process is calculated to be the rate-determining step in both the reactions, and their energy barriers are 28.45 kcal mol−1 associated with the formation of 3,5-disubstituted pyrazole and 31.07 kcal mol−1 associated with the formation of 3,5-disubstituted isoxazole. In particular, the novel bimolecular proton transfer process has reasonably explained in detail on how and why this kind of reaction occurs, and this would provide valuable clues for the rational design of Brønsted acid/base catalysts to promote the synthesis of the five-membered N-heterocyclic compounds.

 Please wait while we load your content...
Please wait while we load your content...