Abstract
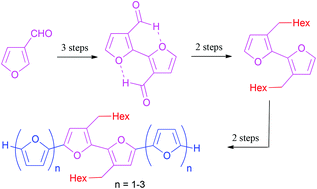
An expedient approach to the synthesis of well soluble symmetrical dialkyl-substituted α-oligofurans containing up to 8 π-conjugated furan heterocycles is reported. An ultimate symmetry and high solubility of these α-oligofurans were guaranteed using the 3,3′-diheptyl-2,2′-bifuran core and its symmetrical elongation through Suzuki–Miyaura or Stille cross-couplings. 3,3′-Diheptyl-2,2′-bifuran was prepared from 2,2′-bifuran-3,3′-dicarbaldehyde by the Wittig olefination and subsequent Pd/C-catalyzed transfer hydrogenation. The most appropriate access to 2,2′-bifuran-3,3′-dicarbaldehyde was achieved through a regioselective lithiation of 3-furanaldehyde acetal followed by CuCl2-induced homocoupling and deprotection. Single crystal X-ray analysis of 2,2′-bifuran-3,3′-dicarbaldehyde revealed anti-arrangement of the furan rings in planar molecules and an unexpected tight herringbone-type packing in crystals.

 Please wait while we load your content...
Please wait while we load your content...