A versatile synthesis of “tafuramycin A”: a potent anticancer and parasite attenuating agent†
Abstract
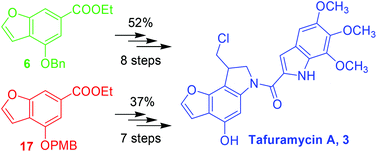
An improved and versatile synthesis of tafuramycin A, a potent anticancer and parasite-attenuating agent, is reported. The three major improvements that optimized yield, simplified purification and allowed the synthesis of more versatile duocarmycin analogues are: a first-time reported regioselective bromination using DMAP as catalyst; the control of the aryl radical alkene cyclization step to prevent the dechlorination side reaction; and the design of a new protection/deprotection method to avoid furan double bond reduction during the classical O-benzyl deprotection in the final step. This alternative protection/deprotection strategy provides ready access to duocarmycin seco-analogues that carry labile functionalities under reducing reaction conditions. Tafuramycin A (3) was prepared in either 8 steps from intermediate 6 or 7 steps from intermediate 17 in 52% or 37% yield respectively. Our strategy provides a significant improvement on the original procedure (11% overall yield) and greater versatility for analogue development.

 Please wait while we load your content...
Please wait while we load your content...