Tri-isopropylsilyl thioglycosides as masked glycosyl thiol nucleophiles for the synthesis of S-linked glycosides and glyco-conjugates†
Abstract
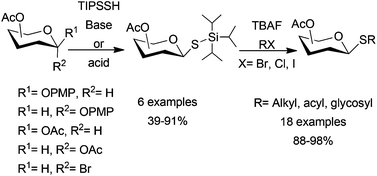
Tri-isopropylsilyl thio-glycosides (TIPS S-glycosides) were synthesized through base promoted SN2 substitution of glycosyl halides with TIPS-SH or by Lewis acid promoted glycosylation of TIPS-SH with glycosyl acetates or p-methoxyphenyl glycosides. Various thioglycoside derivatives were obtained in high yields by one-pot fluoride-mediated de-silylation and thiol alkylation with alkyl halides or Michael acceptors of one common TIPS S-glycoside.

 Please wait while we load your content...
Please wait while we load your content...