Organocatalytic enantioselective allylic alkylation of MBH carbonates with β-keto esters†
Abstract
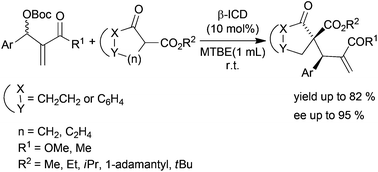
The highly stereoselective allylic alkylation of Morita–Baylis–Hillman carbonates with β-ketoesters catalysed by β-ICD is described. The corresponding products containing two adjacent quaternary and tertiary carbon centers were obtained in good yields with high diastereoselectivity (up to 10 : 1 dr) and enantioselectivity (up to 95% ee).

 Please wait while we load your content...
Please wait while we load your content...