Enantioselective one-pot synthesis of dihydroquinolones via BINOL-derived Lewis acid catalysis†
Abstract
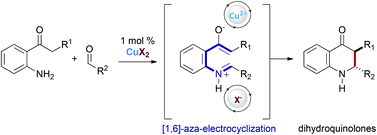
A high-yielding and diastereoselective route to biologically significant 2-aryl- and 2-alkyl-3-amido dihydroquinolones has been developed in up to 90 : 10 e.r. by employing a novel Lewis acidic BINOL-derived copper(II) catalyst.

 Please wait while we load your content...
Please wait while we load your content...