Palladium-catalyzed tandem reaction of 2-hydroxyarylacetonitriles with sodium sulfinates: one-pot synthesis of 2-arylbenzofurans†
Abstract
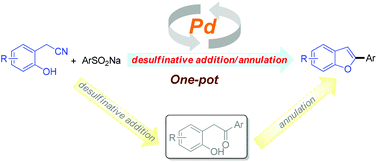
The first example of the palladium-catalyzed one-pot synthesis of 2-arylbenzofurans in moderate to excellent yields via a tandem reaction of 2-hydroxyarylacetonitriles with sodium sulfinates is reported. A plausible mechanism for the formation of 2-arylbenzofurans involving desulfinative addition and intramolecular annulation reactions is proposed. Moreover, the present synthetic route to benzofurans could be readily scaled up to the gram quantity without any difficulty. Thus, the method represents a convenient and practical strategy for synthesis of benzofuran derivatives.

 Please wait while we load your content...
Please wait while we load your content...