Chiral Brønsted acid catalyzed enantioselective intermolecular allylic aminations†
Abstract
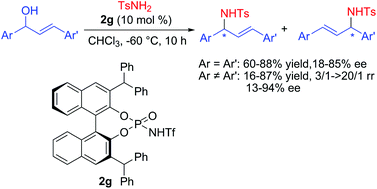
This paper describes an enantioselective intermolecular allylic amination catalyzed by a chiral Brønsted acid via a possible chiral contact ion pair intermediate. A variety of symmetrical or unsymmetrical allylic alcohols can be smoothly aminated to afford the desired products in moderate to high yields with good enantioselectivities and/or regioselectivities.
 Please wait while we load your content...
Please wait while we load your content...