Studies on the stereochemical assignment of 3-acylidene 2-oxindoles†
Abstract
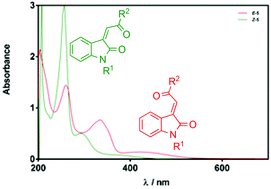
The designation of E/Z-geometrical isomers in 3-acylidene 2-oxindoles by NMR spectroscopy can lead to erroneous assignment of alkene stereochemistry because of the narrow chemical shift range observed over a large series of analogues. In contrast, UV-Vis spectroscopy offers a convenient and more reliable method for alkene stereochemical assignment. A combination of X-ray crystallography and theoretical studies shows that the observed differences in UV-Vis spectroscopic behaviour relate to the twisted conformation of the Z-isomers that provides reduced conjugation and weaker hypsochromic (blue-shifted) absorbances relative to those of the E-isomers.

 Please wait while we load your content...
Please wait while we load your content...