Synthesis of 1,4-dihydroquinoline derivatives under transition-metal-free conditions and their diverse applications†
Abstract
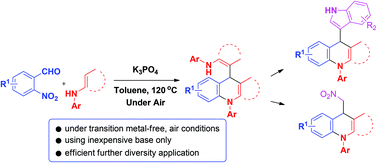
A transition-metal-free process for the synthesis of 1,4-dihydroquinoline derivatives starting from simple enaminones with aldehydes via intermolecular cascade cyclization in a one-pot protocol is developed. This methodology affords a variety of products in moderate to good yields. Particularly, the use of the enaminone fragment in 1,4-dihydroquinoline derivatives 3 as a leaving group for further diverse applications with C-nucleophiles is proved to be feasible.

 Please wait while we load your content...
Please wait while we load your content...