Abstract
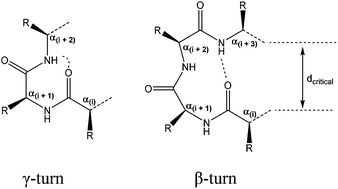
Small peptides are essential mediators of numerous physiological processes. Consequently, there is huge interest in the de novo design of peptides with a predictable folding and related biological activity. In this study, we investigate the possibility of modulating the secondary structure of tetrapeptides through proline N-oxide moieties and N-methylation of the peptide backbone. A series of tetrapeptides were synthesised to investigate the combined effect of Pro N-oxide and N-methylation of the amide bond on the (n + 1) residue in terms of cis- and trans-isomerization, as well as how these modifications direct potential intramolecular hydrogen bonding interactions. The right combination of both these parameters led to a trans to cis-conformational interconversion and a change in the nature of the hydrogen bonding interactions, as demonstrated by NMR spectroscopic, molecular modeling analysis and thermal coefficient studies. Proline N-oxide residues were proposed to induce turns we named as NO-γ-turns and NO-β-turns based on their similarity to traditional γ- and β-turns.

 Please wait while we load your content...
Please wait while we load your content...