Structurally modified natural sesquiterpene lactones constitute effective and less toxic schistosomicidal compounds†
Abstract
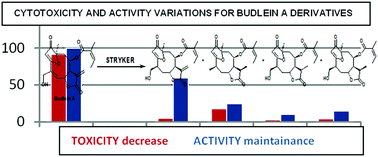
Sesquiterpene lactones are known to be active, but are also known to present high cytotoxicity. In the present work an evaluation of how slight structural alterations affect the cytotoxicity and the schistosomicidal activity of sesquiterpene lactones was undertaken. More specifically, we assessed the activity of budlein-A, a furanoheliangolide sesquiterpene lactone, and four of its derivatives. The structural modifications of budlein-A, presented in this work, diminished the cytotoxicity and changed the antiparasitary behavior of the molecule. They also provided data for a better understanding of the sesquiterpene lactone cytotoxicity. The establishment of the structures of three synthesized sesquiterpene lactones on the basis of NMR and HRESIMS data is also presented here. Complete and detailed 1H and 13C 1D and 2D NMR data, with measurements of all J values and all multiplicities clarified, are presented for five sesquiterpene lactones for the first time.

 Please wait while we load your content...
Please wait while we load your content...