Efficient C(sp3alkyl)–SCF3 bond formations via copper-mediated trifluoromethylthiolation of alkyl halides†
Abstract
A general and convenient copper-mediated trifluoromethylthiolation of primary and secondary alkyl halides was described. Variation of the solvent, additives and time allowed optimization of the reaction. A wide range of alkyl halides were explored to give a set of alkyl trifluoromethyl thioethers in moderate to excellent yields. A variety of functional groups, including ethers, thioether, esters, nitriles, amides, and ketal groups, were well tolerated in the electrophilic partner.
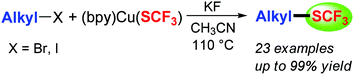

 Please wait while we load your content...
Please wait while we load your content...