Silyl alkynylphosphine-boranes: key precursors of triazolylphosphines via tandem desilylation-Click chemistry†
Abstract
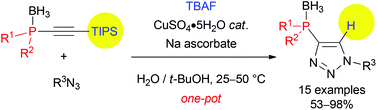
A versatile synthesis of 1,2,3-triazolyl-4-phosphines from the borane complexes of phosphino-alkynes is reported. The efficiency of the procedure relies on the use of readily available silyl-protected alkynylphosphine-boranes, which were subjected to desilylation with TBAF followed by copper-catalyzed azide–alkyne-cycloaddition in one pot. Subsequent treatment with DABCO afforded the targeted triazolylphosphines in high yields. The reported method was applied to the synthesis of the first example of an enantioenriched P-stereogenic triazolylphosphine (98.8% ee).

 Please wait while we load your content...
Please wait while we load your content...