“One-pot” access to dihydrofurans via tandem oxidative difunctionalization and ring contraction of aminopyrans†
Abstract
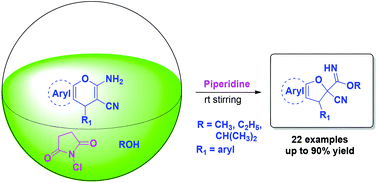
An operationally simple and efficient protocol for the construction of dihydrofuran derivatives has been accomplished via a sequential addition of N-chlorosuccinimide and a base to 2-amino-4H-pyran derivatives in alcohol medium. The one-pot protocol proceeding via tandem oxidative difunctionalization and ring contraction provides an entirely new strategy for the construction of the dihydrofuran skeleton.

 Please wait while we load your content...
Please wait while we load your content...