Phosphodiesters serve as potentially tunable aglycones for fluoro sugar inactivators of retaining β-glycosidases†
Abstract
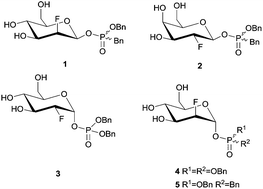
2-Deoxy-2-fluoroglycosides bearing dibenzyl phosphate and phosphonate aglycones were synthesised and tested as covalent inactivators of several retaining α- and β-glycosidases. β-D-Gluco-, -manno- and -galacto-configured benzyl-benzylphosphonate derivatives efficiently inactivated β-gluco-, β-manno- and β-galactosidases, while α-gluco- and α-manno-configured phosphate and phosphonate derivatives served instead as slow substrates.

 Please wait while we load your content...
Please wait while we load your content...