Aryl ethynyl anthraquinones: a useful platform for targeting telomeric G-quadruplex structures†
Abstract
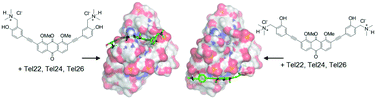
Aryl ethynyl anthraquinones have been synthesized by Sonogashira cross-coupling and evaluated as telomeric G-quadruplex ligands, by the FRET melting assay, circular dichroism, the DNA synthesis arrest assay and molecular docking. Both the binding properties and G-quadruplex vs. duplex selectivity are controlled by the structures of the aryl ethynyl moieties.

 Please wait while we load your content...
Please wait while we load your content...