The asymmetric Cu(ii)–indolinylmethanol complex catalyzed Diels–Alder reaction of 2-vinylindoles with β,γ-unsaturated α-ketoesters: an efficient route to functionalized tetrahydrocarbazoles†
Abstract
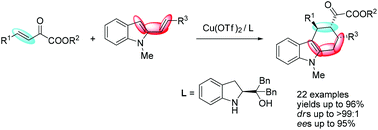
An efficient asymmetric Diels–Alder reaction of 2-vinylindoles with β,γ-unsaturated α-ketoesters has been developed for the construction of functionalized tetrahydrocarbazoles. The products were obtained in high yields (up to 96%) with good stereoselectivities (ee up to 95%, dr up to >99 : 1).

 Please wait while we load your content...
Please wait while we load your content...