Fructose controlled ionophoric activity of a cholate–boronic acid†
Abstract
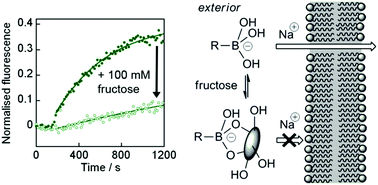
Wulff-type boronic acids have been shown to act as ionophores at pH 8.2 by transporting Na+ through phospholipid bilayers. A cholate–boronic acid conjugate was synthesised and shown to be an ionophore, although the hydroxyl-lined face of the cholate moiety did not enhance ion transport. Mechanistic studies suggested a carrier mechanism for Na+ transport. The addition of fructose (>5 mM) strongly inhibited ionophoric activity of the cholate–boronic acid conjugate, mirrored by a strong decrease in the ability of this compound to partition into an organic phase. Modelling of the partitioning and ion transport data, using a fructose/boronic acid binding constant measured at pH 8.2, showed a good correlation with the extent of fructose/boronic acid complexation and suggested high polarity fructose/boronic acid complexes are poor ionophores. The sensitivity of ion transport to fructose implies that boronic acid-based antibiotic ionophores with activity modulated by polysaccharides in the surrounding environment may be accessible.

 Please wait while we load your content...
Please wait while we load your content...