Improved hemicryptophane hosts for the stereoselective recognition of glucopyranosides†
Abstract
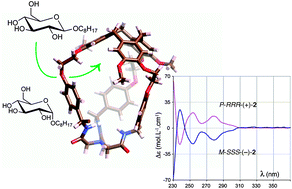
Four new enantiomerically and diastereomerically pure hemicryptophane hosts (M-SSS-2/P-SSS-2 and M-RRR-2/P-RRR-2 pairs) were designed for the recognition of sugar derivatives. Their absolute configuration was determined from the circular dichroism spectra and DFT calculations. The host molecules were then used for the stereoselective recognition of glucopyranosides. Binding constants were obtained from 1H NMR titration experiments showing an increase of affinity for this class of receptors, associated with an improved diastereo- and enantio-differentiation.

 Please wait while we load your content...
Please wait while we load your content...