A Pd-based regioselective strategy to indole-1,2-fused 8- and 9-membered rings: their evaluation as potential scaffolds for apoptosis in zebrafish†
Abstract
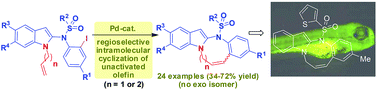
A strategy based on Pd-mediated ring closure of 1,2-disubstituted indoles containing an unactivated olefin leading to indole-1,2-fused 8- and 9-membered rings has been developed for the identification of new and potential scaffolds for apoptosis. A large number of fused indole derivatives containing an endocyclic double bond were synthesized using this robust methodology. A representative compound showed promising apoptotic properties in zebrafish embryos.

 Please wait while we load your content...
Please wait while we load your content...