Bifunctional ferrocene-based squaramide-phosphine as an organocatalyst for highly enantioselective intramolecular Morita–Baylis–Hillman reaction†
Abstract
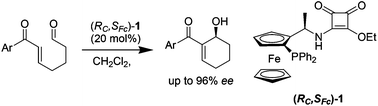
This work demonstrates that, in accord with metal catalysis, ferrocene could be an excellent scaffold for organocatalysts. The simple and easily accessible bifunctional ferrocene-based squaramide-phosphine shows high enantioselectivity in the intramolecular Morita–Baylis–Hillman reaction of 7-aryl-7-oxo-5-heptenals, giving a variety of 2-aroyl-2-cyclohexenols in up to 96% ee.

 Please wait while we load your content...
Please wait while we load your content...