Chemoselective and repetitive intermolecular cross-acyloin condensation reactions between a variety of aromatic and aliphatic aldehydes using a robust N-heterocyclic carbene catalyst†
Abstract
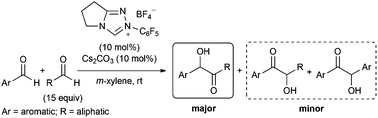
We found that chemoselectivity of the crossed acyloin product is controlled by the adjustment of the aromatic aldehyde/aliphatic aldehyde ratio. Moreover, we observed the persistent catalytic activity of the homogeneous NHC catalyst in a solution due to NHC catalyst robustness.

 Please wait while we load your content...
Please wait while we load your content...