Towards the diastereoselective synthesis of derivative of 11′-epi-brevipolide H†
Abstract
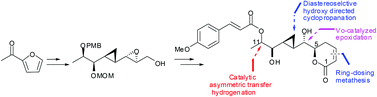
An efficient diastereoselective synthesis of brevipolide H derivative is described. The approach features the use of (i) catalytic asymmetric transfer hydrogenation, (ii) hydroxyl-directed cyclopropanation, and (iii) substrate-controlled catalytic epoxidation and ring-closing metathesis. Remarkably, in this convergent synthesis process, stereogenic centers were installed through catalytic reactions with high stereocontrol, which greatly facilitates the synthesis of stereo-divergent derivatives.

 Please wait while we load your content...
Please wait while we load your content...