The first convergent total synthesis of penarolide sulfate A2, a novel α-glucosidase inhibitor†
Abstract
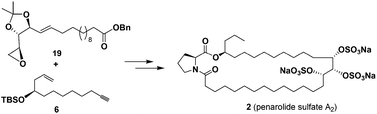
Penarolide sulfate A2, a 31-membered macrolide encompassing a proline residue and three sulfate groups, was firstly synthesized in 16 linear steps with 4.8% overall yield. Three consecutive stereogenic centers in penarolide sulfate A2 were efficiently derived from natural chiral template L-arabinose. The crucial assembly reactions included Brown asymmetric allylation, olefin cross-metathesis, alkyne-epoxide coupling, and macrolactamization. The anti-yeast α-glucosidase activities of penarolide sulfate A2 and its fully desulfated derivative were examined showing IC50 values of 4.87 and 10.74 μg mL−1, respectively.

 Please wait while we load your content...
Please wait while we load your content...