Highly diastereoselective addition of alkoxyethynyl aluminium reagents to N-tert-butylsulfinyl aldimines†
Abstract
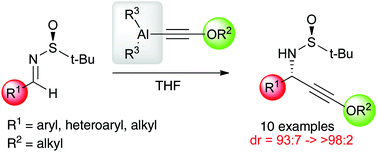
The addition of dimethylaluminium alkoxyacetylides to Ellman's sulfinylimines is described. The reaction proceeds with excellent diastereoselectivity and high yield to produce oxygenated propargylamines in one step starting from simple dichloroenol ethers.

 Please wait while we load your content...
Please wait while we load your content...