Stable selones in glutathione-peroxidase-like catalytic cycle of selenonicotinamide derivative†
Abstract
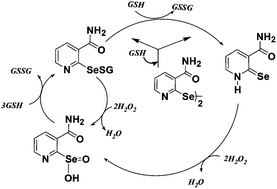
Selenonicotinamide, 2,2′-diselenobis[3-amidopyridine] (NictSeSeNict) exhibits glutathione-peroxidase (GPx)-like activity, catalyzing the reduction of hydrogen peroxide (H2O2) by glutathione (GSH). Estimated reactivity parameters for the reaction of selenium species, according to the Dalziel kinetic model, towards GSH (ϕGSH) and H2O2 (ϕH2O2), indicated that the rate constant for the reaction of NictSeSeNict with GSH is higher as compared to that with H2O2, indicating that the activity is initiated by reduction. 77Se NMR spectroscopy, HPLC analysis, mass spectrometry (MS) and absorption spectroscopy were employed to understand the nature of selenium intermediates responsible for the activity. The 77Se NMR resonance at 525 ppm due to NictSeSeNict disappeared in the presence of GSH with the initial appearance of signals at δ 364 and 600 ppm, assigned to selone (NictC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Se) and selenenyl sulfide (NictSeSG), respectively. Reaction of H2O2 with NictSeSeNict produced a mixture of selenenic acid (NictSeOH) and seleninic acid (NictSeO2H) with 77Se NMR resonances appearing at 1069 and 1165 ppm, respectively. Addition of three equivalents of GSH to this mixture produced a characteristic 77Se NMR signal of NictSeSG. HPLC analysis of the product formed by the reaction of NictSeSeNict with GSH confirmed the formation of NictC
Se) and selenenyl sulfide (NictSeSG), respectively. Reaction of H2O2 with NictSeSeNict produced a mixture of selenenic acid (NictSeOH) and seleninic acid (NictSeO2H) with 77Se NMR resonances appearing at 1069 and 1165 ppm, respectively. Addition of three equivalents of GSH to this mixture produced a characteristic 77Se NMR signal of NictSeSG. HPLC analysis of the product formed by the reaction of NictSeSeNict with GSH confirmed the formation of NictC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Se absorbing at 375 nm. Stopped-flow kinetic studies with global analysis revealed a bimolecular rate constant of 4.8 ± 0.5 × 103 M−1 s−1 and 1.7 ± 0.6 × 102 M−1 s−1 for the formation of NictC
Se absorbing at 375 nm. Stopped-flow kinetic studies with global analysis revealed a bimolecular rate constant of 4.8 ± 0.5 × 103 M−1 s−1 and 1.7 ± 0.6 × 102 M−1 s−1 for the formation of NictC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Se produced in two consecutive reactions of NictSeSeNict and NictSeSG with GSH, respectively. Similarly the rate constant for the reaction of NictC
Se produced in two consecutive reactions of NictSeSeNict and NictSeSG with GSH, respectively. Similarly the rate constant for the reaction of NictC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Se with H2O2 was estimated to be 18 ± 1.8 M−1 s−1. These studies clearly indicated that the GPx activity of NictSeSeNict is initiated by reduction to form NictSeSG and a stable selone, which is responsible for its efficient GPx activity.
Se with H2O2 was estimated to be 18 ± 1.8 M−1 s−1. These studies clearly indicated that the GPx activity of NictSeSeNict is initiated by reduction to form NictSeSG and a stable selone, which is responsible for its efficient GPx activity.

 Please wait while we load your content...
Please wait while we load your content...