Enantioselective organocatalytic oxa-Michael addition of oximes to β-CF3-β-disubstituted nitroalkenes: efficient synthesis of β-amino-α-trifluoromethyl alcohols†
Abstract
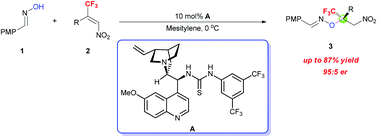
An enantioselective oxa-Michael addition of oximes to β-CF3-β-disubstituted nitroalkenes catalyzed by a chiral bifunctional cinchona alkaloid-based thiourea has been developed. A variety of trifluoromethylated oxime ethers possessing a tetrasubstituted carbon stereocenter were obtained in good yields with high enantioselectivities.

 Please wait while we load your content...
Please wait while we load your content...