Sequential stereodivergent organocatalysis and programmed organocascades†
Abstract
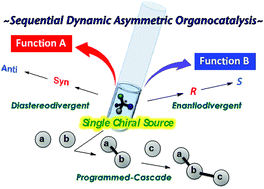
Asymmetric organocatalysis has attracted great interest as a synthetic strategy during the past decade. But, although the inertness of organocatalysts to moisture and oxygen offers great opportunities to tune the reaction conditions, the stereoswitchable character of organocatalysts has not been systematically studied, and most findings have been serendipitous. In this Perspective, we emphasize the importance of in situ tunability in dynamic asymmetric organocatalysis for obtaining different functional outcomes with single-flask operation.

 Please wait while we load your content...
Please wait while we load your content...