Diversity oriented synthesis of novel haloglycolipids potentially useful for crystallization of integral membrane proteins†
Abstract
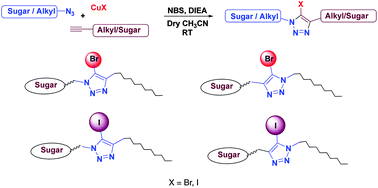
A series of novel haloglycolipids were synthesized based on Cu(I) catalyzed Huisgen's [3 + 2] cycloaddition reaction of diversely functionalized azides and alkynes, using a mixture of N-bromosuccinimide and Cu(I) halide as the halogen source. Since halogen atoms, like bromine and iodine, with a very high scattering power facilitate the solving of crystal structures, the title haloglycolipids could prove to be invaluable in structure-based drug design involving membrane proteins as targets.

 Please wait while we load your content...
Please wait while we load your content...