Targeting abasic site-containing DNA with annelated quinolizinium derivatives: the influence of size, shape and substituents†
Abstract
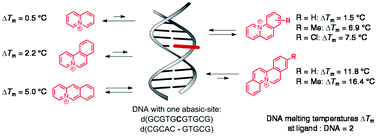
The interactions of regular DNA and abasic site-containing DNA (AP-DNA) with quinolizinium (1a), the linearly fused benzo[b]quinolizinium (2a), the angularly fused benzo[a]quinolizinium (3a), benzo[c]quinolizinium (4a), and dibenzo[a,f]quinolizinium (5a) as well as derivatives thereof were studied with photometric and viscosimetric titrations (regular DNA), fluorimetric titrations and thermal DNA denaturation experiments (regular DNA and AP-DNA). Whereas the parent quinolizinium ion (1a) and the benzo-annelated derivatives 2a, 3a and 4a exhibit no significant affinity to AP-DNA, additional benzo-annelation in 5a leads to an increased selective stabilization of AP-DNA by this ligand. Hence, the latter compound represents the first example of a ligand that does not require ancillary substituents for efficient AP-DNA stabilization. In addition, studies of derivatives with varied substitution patterns revealed an impact of substituents on the stabilization of the AP-DNA. We discovered that a chloro substituent affects the propensity of a ligand to bind to AP-DNA in a similar way as the methyl substituent and may be employed complementary to the known methyl effect to increase the binding affinity of a ligand.

 Please wait while we load your content...
Please wait while we load your content...