A highly efficient in situ N-acetylation approach for solid phase synthesis†
Abstract
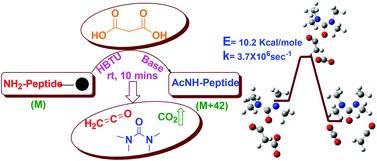
We describe a new general N-acetylation method for solid phase synthesis. Malonic acid is used as a precursor and the reaction proceeds by in situ formation of a reactive ketene intermediate at room temperature. We have successfully applied this methodology to peptides and non-peptidic molecules containing a variety of functional groups. The reaction gave high yields compared to known acetylation methods, irrespective of the structure, conformation and sequence of the acetylated molecule. Computational studies revealed that the concerted mechanism via the ketene intermediate is kinetically favorable and leads to a thermodynamically stable acetylated product. In conclusion, our method can be easily applied to acetylation in a wide variety of chemical reactions performed on the solid phase.

 Please wait while we load your content...
Please wait while we load your content...