Alkoxyamines: a new family of pro-drugs against cancer. Concept for theranostics
Abstract
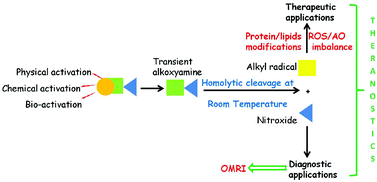
Development of anti-cancerous theranostic agents is a vivid field. This article describes a theranostic approach that relies on the triggering of cancer cell death by generation of alkyl radicals at the right place and at the right time using the presence of active proteases in the tumour environment. Alkoxyamines (R1R2NOR3) are labile molecules that homolyze into nitroxides (R1R2NO˙) and reactive alkyl radicals (R3˙). They are used as a source of active alkyl radicals for curing and nitroxides for monitoring by Overhauser-enhanced magnetic resonance imaging (OMRI). Herein, the requirements needed for applying alkoxyamines are described: (i) highly selective activation of the alkoxyamine by specific proteases; (ii) fast homolysis of the alkoxyamine C–ON bond at physiological temperature; (iii) activation of cell death processes through an increase of the local oxidative stress or potential re-activation of the immune system due to short-lived alkyl radicals; and (iv) imaging of the tumor and the drug release by sensing the nitroxide by OMRI.

 Please wait while we load your content...
Please wait while we load your content...