Nickel-catalyzed triarylamine synthesis: synthetic and mechanistic aspects†
Abstract
An improved protocol was described for the amination of chloroarenes with diarylamines under NiCl2(PCy3)2 catalysis in the presence of a Grignard reagent as base. This method fully suits bromo-/iodoarene substrates as well, and even is expanded to certain aryl tosylates. A preliminary investigation into the mechanism suggests that this amination reaction might proceed through NiI and NiIII intermediates rather than via the usually expected Ni0–NiII cycle.
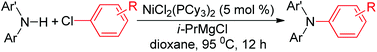

 Please wait while we load your content...
Please wait while we load your content...