NHC-catalyzed oxidative γ-addition of α,β-unsaturated aldehydes to isatins: a high-efficiency synthesis of spirocyclic oxindole-dihydropyranones
Abstract
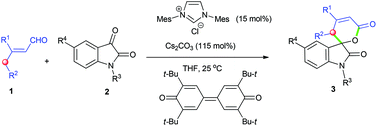
This manuscript discloses an efficient construction of the spirocyclic oxindole-dihydropyranone scaffold via the N-heterocyclic carbene (NHC)-catalyzed oxidative γ-functionalization of α,β-unsaturated aldehydes bearing γ-H with isatin derivatives. The ready availability of the starting materials, easy work-up, mild reaction conditions and the potential utilization value of the products make this strategy attractive.

 Please wait while we load your content...
Please wait while we load your content...