Synthesis of benzosultams via an intramolecular sp2 C–H bond amination reaction of o-arylbenzenesulfonamides under metal-free conditions†
Abstract
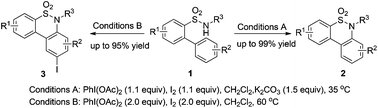
A practical synthetic method for the generation of benzosultams via an intramolecular sp2 C–H bond amination reaction of o-arylbenzenesulfonamides with PhI(OAc)2–I2 under metal-free conditions is developed. A broad range of substrates are tolerated under mild reaction conditions, affording bioactive benzosultams in good to excellent yields. The resulting benzothiazines could be conveniently transformed into their corresponding iodinated derivatives via electrophilic substitution reactions.

 Please wait while we load your content...
Please wait while we load your content...