Tandem diastereo- and enantioselective preparation of aryl and alkyl cyclopropyl carbinols with three adjacent stereocenters using perhydrobenzoxazines and diethylzinc†
Abstract
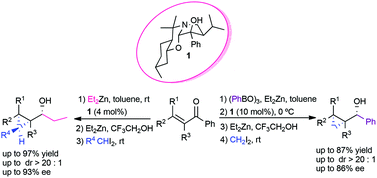
The enantio- and diastereoselective one-pot ethylation/cyclopropanation is efficiently promoted by a chiral perhydrobenzoxazine. The catalytic system tolerates a wide range of di- and trisubstituted α,β-unsaturated aldehydes and has been found to be highly diastereo- and enantioselective. Enals leading to intermediates lacking allylic strain or with either A1,2 or A1,3 strain afford the corresponding syn hydroxycyclopropanes very selectively. While α-methyl enals are successfully ethylated/cyclopropanated, the presence of bulky substituents at the alpha position of the enal constitutes a limitation to the substrate scope. The use of 1,1-diiodoethane allows the obtention of the corresponding enantioenriched cyclopropylcarbinol, which bears carbon-substituents at all three positions of the ring, with good enantiocontrol, although moderate diastereoselectivity. A procedure for the asymmetric one-pot arylation/cyclopropanation of enals is proposed, which involves the use of triarylboroxin, diethylzinc and diiodomethane.

 Please wait while we load your content...
Please wait while we load your content...