Tandem Michael addition/isocyanide insertion into the C–C bond: a novel access to 2-acylpyrroles and medium-ring fused pyrroles†
Abstract
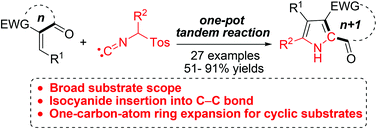
A new reaction model, tandem Michael addition/formal isocyanide insertion into the acyl C–C bond, has been developed. Thus, a series of 2-acylpyrroles and seven-/eight-membered ring fused pyrroles were synthesized from the reaction of methyl isocyanides with enones in a single operation.

 Please wait while we load your content...
Please wait while we load your content...