Approaches to the synthesis of a novel, anti-HIV active integrase inhibitor†
Abstract
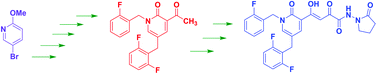
The novel HIV-1 integrase inhibitor 1, discovered in our laboratory, exhibits potent anti-HIV activity against a diverse set of HIV-1 isolates and also against HIV-2 and SIV. In addition, this compound displays low cellular cytotoxicity and possesses a favorable in vitro drug interaction profile with respect to isozymes of cytochrome P450 (CYP) and uridine 5′-diphospho-glucuronosyltransferase (UGT). However, the total synthesis of this significant HIV integrase inhibitor has not been reported. This contribution describes an optimized, reproducible, multi-step, synthetic route to inhibitor 1. The yield for the separate steps averaged about 80%. The methodologies utilized in the synthesis were, among others, a palladium-catalyzed cross-coupling reaction, a crossed-Claisen condensation, and a hydrazino amide synthesis step. Successful alternative synthetic methodologies for some of the steps are also described.

 Please wait while we load your content...
Please wait while we load your content...