Synthesis of PDE IV inhibitors.† First asymmetric synthesis of two of GlaxoSmithKline's highly potent Rolipram analogues‡
Abstract
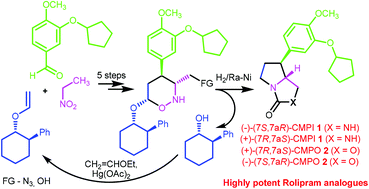
Asymmetric syntheses of two of GlaxoSmithKline's highly potent phosphodiesterase IV inhibitors CMPI 1 and CMPO 2 have been accomplished from nitroethane and simple precursors in 8 and 7 steps, respectively. The suggested synthetic strategy involves as a key stage the silylation of enantiopure six-membered cyclic nitronates. In vitro studies of PDE IVB1 inhibition revealed a significant difference in the activity of CMPI 1 and CMPO 2 enantiomers.

 Please wait while we load your content...
Please wait while we load your content...