Stereospecific synthesis of a twinned alanine ester†
Abstract
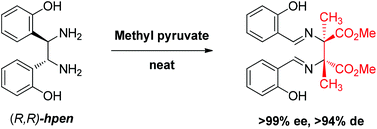
Reaction between 1,2-bis(2-hydroxyphenyl)-ethylenediamine (hpen) and methyl pyruvate gives the diaza-Cope rearrangement product with good yield and excellent stereospecificity. The product containing two chiral quaternary carbon centers is characterized by high performance liquid chromatography and X-ray crystallography. DFT computation provides insight into why the diaza-Cope rearrangement takes place readily with methyl pyruvate but not with other ketones like acetone and substituted acetophenones.

 Please wait while we load your content...
Please wait while we load your content...