Regioselective halogenation of 2-substituted-1,2,3-triazoles via sp2 C–H activation†
Abstract
A highly regioselective halogenation of 2-substituted-1,2,3-triazoles was developed via sp2 C–H activation. This method is compatible with halogen atoms, as well as electron-donating and electron-withdrawing groups. Meanwhile, the strategy is also efficient for the synthesis of a key intermediate of Suvorexant.
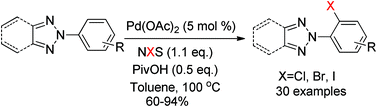

 Please wait while we load your content...
Please wait while we load your content...